4-Iodoaniline
1-Aminopyridinium Iodide
1-Diazo-2-naphthol-4-sulfonic acid
2,2,2-Triphenylacetophenone
2,6-Diamino Toluene
2-Amino-4-chlorobenzonitrile
2-Iodophenol
3,4-Dimethyl Benzophenone
3,5-Dinitroaniline
3,5-Dinitrosalicylic Acid
3-Bromo-2-(bromomethyl)propionic acid
3-Chlorobenzonitrile
4-(2-Chloroethyl) Morpholine HCL
2-(2-Hydroxyethyl) pyridine
4-(Dimethylamino) Benzaldehyde
4-Chloro-2-Nitro Benzonitrile
2-Iodothiophene
Diphenic acid (Biphenyl-2,2'dicarboxylic Acid)
Ethyl 2-(diphenylmethyleneamino)acetate
Methyl Crotonate
Pentaerythritol-Tetrabenzoate
Pentaerythrityl Tetraacetate
Rhodanine
Sodium Sitroprusside Dihydrate
Sulphapyridine
3-Acetamidephenol
Tert-butyl Carbamate
Diphenyl Diselenide
2,2'-Dihydroxy-3,3',5,5' tetra tert butyl biphenyl
Alpha-d-glucofuranose,1,2-0-(2,2,2-trichloroethylidene)-,(r)
1,1-Diphenylacetone
2-(2-hydroxyethyl)-pyridine (Stellar-2056)
3,5-Dinitroaniline (Stellar-2055)
2,4-Dichloro-6-phenyl-1,3,5-triazine (Stellar-2054)
2-Chloro-4-(3-chloro-phenyl)-6-phenyl-1,3,5-triazine (2125473-29-0) (Stellar- 2053)
9-[4,6-Bis[3-(triphenylsilyl)phenyl]-1,3,5-triazin-2-yl]-9H-carbazole (Stellar-2052)
2,4-Diphenyl-6-[3-(triphenylsilyl)phenyl]-1,3,5-triazine (Stellar-2051)
2-Chloro-4,6-bis(dibenzo(b,d)furan-4-yl)-1,3,5-triazine (Stellar-2050)
2-Chloro-4-(dibenzofuran-1-yl)-6-(naphthalen-2-yl) -1,3,5-triazine (Stellar-2049)
2-Chloro-4,6-bis(1-dibenzofuranyl)1,3,5-triazine (2392930-05-9) (stellar - 2048)
2-chloro-4-(dibenzo(b,d)furan-1-yl)-6-phenyl -1,3,5-triazine (Stellar-2047)
2-Chloro-4-(naphthalen-2-yl)-6-phenyl-1,3,5-triazine (Stellar-2046)
2-(4,6-Di-1-naphthalenyl-1,3,5- triazin-2-y-l)-5-{(2-ethylhexyl)oxyphenol (518045-49-3)(stellar-2045)
Bis-naphthyl(2,4-hydroxy-n-hexyloxyphenyl)-1,3,5-triazine (518045-49-3) (stellar-2044)
Bis-naphthyl(2,4-dihydroxyphenyl)-1,3,5-triazine (518045-48-2) (stellar-2043)
2-Chloro-4,6-di-1-naphthalenyl-1,3,5-triazine (78941-32-9) (Stellar-2042)
Choline bicarbonate (Stellar-2041)
1,3-Bis(1,1-dimethylpropyl) dicarbonate (Stellar-2037)
4,4'-Bis(bromomethyl)diphenyl ether (Stellar-2038)
4,4'-Bis(hydroxymethyl)diphenyl ether (Stellar-2039)
Bis(4-chlorophenyl)sulfoxide (Stellar-2040)
4-(2-(Methylamino)ethoxy)aniline (Stellar-2036)
1-Oxo-2,3-dihydro-1H-indene-4-carbonitrile
3,6-Diaminocarbazole (Stellar-2035)
Triallyl Isocyanurate (TAIC) (Stellar-2034)
2-(4-Chloro-3-Nitrobenzoyl) Benzoic Acid
2,4-diamino-6-(2,5-dichlorophenyl)-1,3,5-triazine maleate (appolo-206)
4,4'-Methylene-bis (2-methylaniline) (Stellar-2033)
N-cclopropyl-1,3,5-triazine-2,4,6-triamine (cyromazine) (appolo-205)
Tris (Carboxy methyl) isocyanurate (Stellar-2032)
Methyl 4-(bromomethyl) benzoate
4-(4,6-DIMETHOXY-1,3,5-TRIAZIN-2-YL)-4-METHYL MORPHOLINIUM CHLORIDE (DMTMM)(APPOLO-202)
Pentaerythritol Tetrabenzoate (Stellar-2031)
1,2,4 triazole (P) (Stellar-2030)
2-Iodoxybenzoic acid
3-Bromo-3'-iodo-1,1'- Biphenyl (Stellar-2029)
Diphenylamine
2-Bromofluorenone (Stellar-2028)
Aminodiphenylmethane (BENZHYD)
2,5-dihydroxybenzaldehyde (Stellar-2027)
2-Chloro-4,6-bis[1,1':3',1'']terphenyl-5'-yl-1,3,5-triazine (Stellar-2025)
2,4,6-Tris(2-hydroxy-4-butoxyphenyl)-1,3,5-triazine (Stellar-2026)
4-Methoxybenzylamine
2-Chloro-4,6-di(naphthalen-2-yl)-1,3,5-triazine (Stellar-2024)
3-Iodoaniline
2,7-Dibromo-9-fluorenone (Stellar-2023)
3-Methyl-1-phenyl-2-pyrazolin-5-one
1,3,5-triazine-2,4,6-triyl)tris(benzene-4,1-diyl)tris(ethane-2,1-diyl)triacetate (Appolo-567)
1,3-Propanediol-di-p-tosylate (Stellar-2022)
1,3,5-Tris(4-tert- butyl-3-hydroxy-2,6-dimethyl benzyl)1,3,5-triazine-(1H,3H,5H)-trione (Appolo-1790) [Under Development]
2-Acetamidophenol
Bis(4-iodophenyl) sulfoxide (Stellar-2021)
2,4-Bis-(octylthio)-6-(3,5-di tert butyl-4-hydroxyanilino)-1,3,5-triazine (Appolo-565) [Under Development]
4-(2-Hydrazino-2-oxoethyl)-4-methylmorpholine-4-ium chloride (HMMC)
4,4'-Dinitrodiphenic acid (Stellar-2020)
Ethyl 4-bromophenyl acetate (EBPA)
3,6,9,15-Tetraazabicyclo[9.3.1]pentadeca-1(15), 11,13-triene, Trihydrobromide
2-(Biphenyl-4-yl)-4-chloro-6-phenyl-1,3,5-triazine (Stellar-2019)
2,4-Bis(2,4-dimethylphenyl)-6-(2-hydroxy-4-(2-hydroxyethoxy)phenyl)-1,3,5-triazine (Appolo-1166) [Under Development]
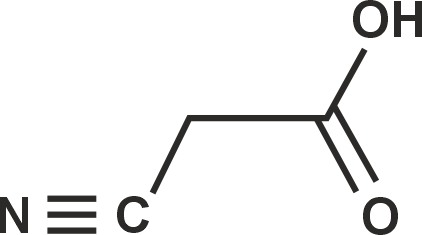
Cyno acetic acid
1,3,5-Tripropyl-1,3,5-triazinane-2,4,6-trione (Stellar-2018)
2,4-Bis-(2,4-dimethyl phenyl)-6-(2-hydroxy-4-hexyloxyphenyl)-1,3,5-triazine (Appolo-1163) [Under Development]
2,4-Dichloro-6-(4-methoxyphenyl)-1, 3,5-triazine (Appolo-122)
2-(2-HYDROXYETHYL)PYRIDINE
4,4-Methylenebis (2,6-dimethylaniline) (Stellar-2017)
2-(4,6-diphenyl-1,3,5-triazin-2-yl)-5-((2-ethylhexyl)oxy) phenol (Appolo-1580) [Under Development]
2-(4-Methoxyphenyl)4,6-bis-(2,4-dihydroxyphenyl) -1,3,5-triazine (Appolo-125 (T))
4-Aminobenzoic acid
2-(2,4-Dihydroxyphenyl)-4,6-diphenyl-1,3,5-triazine (Stellar-2016)
2,4-Bisphenyl-6-(2-hydroxy-4-n-octyloxyphenyl)-1,3,5-triazine (Appolo-1578) [Under Development]
2-(4-Methoxyphenyl)4,6-bis-(2,4-dihydroxyphenyl) -1,3,5-triazine (Appolo-125 (P))
2-Chloro-4,6-Bis(phenyl)-1,3,5-triazine (Stellar-2015)
2,4-Diphenyl-6-hydroxy-1,3,5-triazine (Appolo-114)
2-Ethylhexyl-4-aminobenzoate (Etoneamine) (Sarasorb ETN)
Pyridinium p-toluenesulfonate
N-Ethyl-p-toluidine (Stellar-2014)
2-(2-Hydroxy-4-ethoxyphenyl)-4,6-bis(phenyl)-1,3,5-triazine (Appolo-117)
4,4,4"-(1,3,5-triazine-2,4,6-triytris(2-methylbenzene-1,3-diol (Appolo-461)
Homosalate (Sarasorb HMS)
4-Amino-6-(trifluoromethyl)benzene-1,3-disulfonamide (TFMSAA)
2-NITROTHIOPHENE (Stellar-2013)
2-2,4-Dihydroxyphenyl-4,6-diphenyl-1,3,5-triazine (Appolo-116)
1,3- Benzenediol 4,4,4-(1,3,5 triazine)-2,4,6 tris (Appolo-459)
Ethylhexyl salicylate (Sarasorb EHS)
5-Methoxy-2-nitroaniline (Stellar-2012)
2,6-Dichloroisonicotinic Acid (2,6-Dichloropyridine-4-carboxylic acid)
2-Chloro-4,6-Bis(phenyl)-1,3,5-triazine (Appolo-115)
2,4-Bis(2,4-dimethylphenyl)-6-(2,4-dihydroxyphenyl)-1,3,5-triazine (Appolo-107)
Ethylhexyl methoxycinnamate (Sarasorb OMC)
Benzoic Hydrazide
4-Glycidyloxy benzophenone (Stellar-2011)
2,4-Bis(2,4-dimethylphenyl)-6-(2-hydroxy-4-methyl acetoxy)-1,3,5-triazine (Appolo-1165)
Avobenzone (Sarasorb AVB)
6-Nitro-7-chloro-4-hydroxy quinazoline
9-Fluorenone (Stellar-2010)
2-(4,6-Diphenyl-1,3,5-triazin-2-yl)-5-[2-(2-ethylhexanoyloxy)ethoxy]phenol (Appolo-46)
Bis(2-(4-(4,6-bis(2,4-dimethylphenyl)-1,3,5-triazin-2-yl)-3-hydroxphenoxy)ethyl) dodecanedioate (Appolo-1100)
Terephthalylidene dicamphor sulfonic acid (Sarasorb TDSA)
1-Phenyl-1,2-propanedione - Saracure PPD
3-Bromoacetylpyridine HBr
9-Fluorenone-1-carboxylic acid (Stellar-2009)
Bis(2-[4-(4,6-diphenyl-1,3,5-triazine-2-yl)-3-hydoxyphenoxyl]ethyl]dodacenedioate (Appolo-1000)
2,4,6-Tris(2-hydroxy-4-butoxyphenyl)-1,3,5-triazine & 4-[4,6-Bis(4-butoxy-2-hydroxyphenyl) -1,3,5-triazin-2-yl]-1,3-benzenediol (Appolo-480)
Phenylbenzimidazole Sulfonic Acid (Sarasorb PBSA)
2(Dimethylamino)-2-(4-methylbenzyl)-1 (4-morpholinophenyl)butan-1-one) - Saracure 379
4-Acetylbenzonitrile
Diphenic Acid (Biphenyl-2,2'dicarboxylic Acid) (Stellar-2008)
2,4-Bis(2,4-dimethyl phenyl)-6-(2-hydroxy-4-methoxy phenyl)1,3,5-triazine (Appolo-1164 GL)
2,4,6-Tris (2-hydroxy-4-hexyloxy-3-methylphenyl) -1,3,5-triazine (Appolo-462)
Methylene Bis-Benzotriazolyl Tetramethylbutylphenol (and) Aqua (and) Decyl Glucoside (and) Propylene Glycol (and) Xanthan Gum (Sarasorb M Aqua)
Sarafast UVT
Phenylbis(2,4,6-trimethylbenzoyl)phosphine Oxide - Saracure 819
Benzophenoneimine
Benzothiophene-2-carboxylic acid (Stellar-2006)
2,4-Bis(2,4-dimethylphenyl)-6-(2-hydroxy-4-octyloxyphenyl)-1,3,5-triazine (Appolo-1164 (M))
Zeron 1613
2,4-Bis(2-hydroxy-4-butyloxyphenyl)-6-(2,4-bis -butyloxyphenyl)-1,3,5-triazine (Appolo-460)
2,2′-Methylenebis[6-(2H-benzotriazol-2-yl)-4-(1,1,3,3 -tetramethylbutyl)phenol] (Sarasorb M (MBBT))
Sarafast TR LIQ
2-Hydroxy-4'-(2-hydroxyethoxy)-2-methylpropiophenone - Saracure 2959
Methyl 4-bromocrotonate
4-Benzoylbutyric acid (Stellar-2005)
2,4-Bis(2,4-dimethylphenyl)-6-(2-hydroxy-4-octyloxyphenyl)-1,3,5-triazine (Appolo-1164)
Anthranilamide
1,3,5-tris-[2,2-dimethylpropionylamino]benzene (Saraclear XT 386)
2-[2-Hydroxy-4-[3-(2-ethylhexyl-1-oxy)-2-hydroxypropyloxy]phenyl]-4,6-bis (2,4-dimethylphenyl)-1,3,5-triazine2-[2-Hydroxy-4-[3-(2-ethylhexyl-1-oxy)-2-hydroxypropyloxy]phenyl]-4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine (Appolo - 405)
2,4-Bis(2-hydroxy-4-methoxyphenyl)-6-ethyl mercaptan-1,3,5-triazine (Appolo-124)
Diethylhexylbutamidotriazone (Sarasorb DHBT)
Sarafast SUN PES
2,4-Dimethylpyrrole (Stellar-2004)
2-hydroxy-1-[4-[4-(2-hydroxy-2-methylpropanoyl)phenoxy]phenyl]-2-methylpropan -1-one - Saracure 160
2-(2-Hydroxy-4-hexyloxyphenyl)-4,6-Bis(phenyl)-1,3,5-triazine (Appolo-1577 (Granules))
2-(4,6-bis(2,4-dimethylphenyl)-1,3,5-triazin-2-yl)-5 -((2-ethylhexyl)oxy)phenol (Appolo-1164L)
2-(2-Hydroxy-4-methoxyphenyl)-4,6-Bis(phenyl)-1,3,5-triazine (Appolo-1579 (A-103))
Diethylamino Hydroxybenzoyl Hexyl Benzoate (Sarasorb DHHB)
Sarafast P
Camphorquinone
1-Phenyl-1-cyclopentanecarboxylic acid
2-Iodobenzoic acid (Stellar-2003)
2-(2-Hydroxy-4-hexyloxyphenyl)-4,6-Bis(phenyl)-1,3,5-triazine (Appolo-1577 (FLK))
5-[4-(Dimethylaminobenzylidene)barbituric acid] (Saralite RL-1000 (DMBA))
2,4,6-Tris[4-(1-octyloxycarbonyl)ethyloxy-2-hydroxyphenyl]-1,3,5-triazine (Appolo - 477)
2,4-Bis(2'-hydroxyphenyl)-6-phenylamino-s-triazine (Appolo-425)
2-ethylhexyl-4-[[4,6-bis[4-(2-ethylhexoxycarbonyl)anilino]-1,3,5-triazin-2-yl]amino]benzoate (Sarasorb EHT)
Sarafast HLF NEW
2,4-Thiazolidinedione (P)
2(4-Chlorobenzoyl) Benzoic Acid
2-Acetylthiophene (Stellar-2002)
2-2-Hydroxy-4-hexyloxyphenyl-4,6-Bis(phenyl)-1,3,5-triazine (Appolo-1577)
2-[4-[2-hydroxy-3-dodecyloxypropyl)oxy]-2-hydroxyphenyl]-4,6-bis (2,4-dimethylphenyl-1,3,5-triazine & 2-[4-(2-Hydroxy-3-tridecyloxypropyl)oxy]-2-hydroxyphenyl-4,6-bis(2,4-dimethylphenyl-1,3,5-triazine (Appolo-400)
2-(4-(4-Methoxyphenyl)-6-phenyl-1,3,5-triazine-2-yl) phenol (Appolo-325 70%)
2,4-Bis[4-(2-ethylhexyloxy)-2-hydroxyphenyl]-6 -(4-methoxyphenyl)-1,3,5-triazine (Sarasorb BEMT)
Sarafast AS NEW LIQ
5-Ethyl pyridine-2-ethanol
Homophthalic Acid(Stellar-2001)
2-(4-phenylbenzoyl) Benzoic Acid
4-4-methylenebis[3-chloro-2-6-diethylbenzenamine]
LIST OF UV FILTERS FOR PERSONAL CARE
INTERMEDIATE FOR UV FILTERS
PRODUCTS FOR HAIR CARE
ANTI-MICROBIAL AGENTS
4-Aminoacetanilide
N-heptylamine
Please Select Country *
Afghanistan
Albania
Algeria
Andorra
Angola
Antigua and Barbuda
Argentina
Armenia
Australia
Austria
Azerbaijan
The Bahamas
Bahrain
Bangladesh
Barbados
Belarus
Belgium
Belize
Benin
Bhutan
Bolivia
Bosnia and Herzegovina
Botswana
Brazil
Brunei
Bulgaria
Burkina Faso
Burundi
Cabo Verde
Cambodia
Cameroon
Canada
Central African Republic
Chad
Chile
China
Colombia
Comoros
Congo, Democratic Republic of the
Congo, Republic of the
Costa Rica
Côte d’Ivoire
Croatia
Cuba
Cyprus
Czech Republic
Denmark
Djibouti
Dominica
Dominican Republic
East Timor
Ecuador
Egypt
El Salvador
Equatorial Guinea
Eritrea
Estonia
Ethiopia
Fiji
Finland
France
Gabon
The Gambia
Georgia
Germany
Ghana
Greece
Grenada
Guatemala
Guinea
Guinea-Bissau
Guyana
Haiti
Honduras
Hungary
Iceland
India
Indonesia
Iran
Iraq
Ireland
Israel
Italy
Jamaica
Japan
Jordan
Kazakhstan
Kenya
Kiribati
Korea, North
Korea, South
Kosovo
Kuwait
Kyrgyzstan
Laos
Latvia
Lebanon
Lesotho
Liberia
Libya
Liechtenstein
Lithuania
Luxembourg
Macedonia
Madagascar
Malawi
Malaysia
Maldives
Mali
Malta
Marshall Islands
Mauritania
Mauritius
Mexico
Micronesia, Federated States of
Moldova
Monaco
Mongolia
Montenegro
Morocco
Mozambique
Myanmar (Burma)
Namibia
Nauru
Nepal
Netherlands
New Zealand
Nicaragua
Niger
Nigeria
Norway
Oman
Pakistan
Palau
Panama
Papua New Guinea
Paraguay
Peru
Philippines
Poland
Portugal
Qatar
Romania
Russia
Rwanda
Saint Kitts and Nevis
Saint Lucia
Saint Vincent and the Grenadines
Samoa
San Marino
Sao Tome and Principe
Saudi Arabia
Senegal
Serbia
Seychelles
Sierra Leone
Singapore
Slovakia
Slovenia
Solomon Islands
Somalia
South Africa
Spain
Sri Lanka
Sudan
Sudan, South
Suriname
Swaziland
Sweden
Switzerland
Syria
Taiwan
Tajikistan
Tanzania
Thailand
Togo
Tonga
Trinidad and Tobago
Tunisia
Turkey
Turkmenistan
Tuvalu
Uganda
Ukraine
United Arab Emirates
United Kingdom
United States
Uruguay
Uzbekistan
Vanuatu
Vatican City
Venezuela
Vietnam
Yemen
Zambia
Zimbabwe